UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
Sagimet Biosciences Inc.
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trade Symbol(s) |
Name of each exchange on which registered |
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 | Regulation FD Disclosure |
On September 27, 2023, Sagimet Biosciences Inc. (the “Company”) updated information reflected in a slide presentation, which is attached as Exhibit 99.1 hereto and incorporated herein by reference. The Company will use the updated presentation in various meetings with investors from time to time.
The information in Item 7.01 of this Current Report on Form 8-K, including the information set forth in Exhibit 99.1, is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), nor shall Exhibit 99.1 filed herewith be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01 | Other Events. |
On September 27, 2023, the Company issued a press release announcing that its license partner, Ascletis Bioscience Co. Ltd., has enrolled 120 patients in its Phase 3 registration clinical trial of denifanstat combined with bevacizumab for treatment of recurrent glioblastoma. A copy of this press release is attached as Exhibit 99.2 hereto and incorporated herein by reference.
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits
| Exhibit No. | Document | |
| 99.1 | Investor Presentation of Sagimet Biosciences Inc., dated September 27, 2023 | |
| 99.2 | Press Release of Sagimet Biosciences Inc., dated September 27, 2023. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Sagimet Biosciences Inc. | ||
| Date: September 27, 2023 | By: | /s/ David Happel |
| David Happel | ||
| Chief Executive Officer | ||
Exhibit 99.1

Targeting Metabolic Dysfunction with Novel Therapies to Treat NASH, Acne and Cancer September 27 , 2023

Forward Looking Statements 2 • This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, market opportunity, competitive position and potential growth opportunities are forward - looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements. In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “would,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “believe,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other similar expressions. The forward - looking statements in this presentation are only predictions. These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others: the clinical development and therapeutic potential of denifanstat or any other drug candidates we may develop; our ability to advance drug candidates into and successfully complete clinical trials, including our FASCINATE - 2 Phase 2b clinical trial; our relationship with Ascletis, and the success of its development efforts for denifanstat; the accuracy of our estimates regarding our capital requirements; and our ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission and available at www.sec.gov . You should not rely on these forward - looking statements as predictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements. Moreover, we operate in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

Dave Happel President & CEO • Cognoa: President & CEO Chrono Therapeutics: President & CEO Senior executive and commercial roles at Horizon, Raptor, Dynavax, Chiron • M.B.A. – Indiana State University; B.A. chemistry – Indiana University George Kemble Executive Chairman • AstraZeneca (formerly MedImmune, Aviron): SVP research for biologics and general manager of California operations, VP vaccine research & development for vaccines • Ph.D. – Stanford University, dept of microbiology & immunology Eduardo Martins CMO • Abbvie (formerly Allergan), Eiger BioPharmaceuticals, Gilead, Genentech, Dynavax, Intermune, SciClone • D.Phil. – University of Oxford M.D. – Federal University of Rio de Janeiro, Brazil Anthony Rimac CFO • Cognoa, ESCAPE Bio, Chrono Therapeutics, Aldea Pharmaceuticals, Adamas Pharmaceuticals, Aerovance • M.B.A. – Santa Clara University; B.A. – University of California Santa Barbara Elizabeth Rozek General Counsel and CCO • Cognoa, Basilea Pharmaceutica, US Department of Justice • J.D. – University of California Berkeley M.A. – University of California San Diego B.A. – Brown University Proven Team with Development and Commercialization Experience Across Hepatology, Metabolic Disease and Oncology 3

Sagimet Investment Highlights Critical role of FASN enzyme in NASH x Key enzyme in de novo lipogenesis – responsible for excess liver fat in NASH x FASN inhibition directly improves the 3 key drivers of NASH – liver fat, inflammation, fibrosis x Differentiated MOA to treat growing underserved patient population x FASCINATE - 2 Phase 2b interim data • Statistically significant improvements in key biomarkers of NASH: liver fat, inflammation, fibrosis • Results consistent with Phase 2a study • Strengthen belief in Phase 2b liver biopsy results expected in 1Q 2024 x Blood test confirms drug response x Predictive biomarkers identify likely responders x Opportunity to personalize treatment and optimize outcomes Acne x Clinical proof of concept established in Phase 1 • Positive Phase 2 topline results announced in May 2023 by Ascletis Cancer x Clinical proof of concept established in Phase 1 • Phase 3 rGBM trial enrollment for interim analysis completed in September 20 23 by Ascletis Denifanstat: FASN inhibitor with compelling clinical data Precision medicine is key differentiator Strong rationale for FASN in acne and cancer Strong financial position x Upsized IPO completed in July 2023 raised $96.4 million of gross proceeds x Cash and equivalents expected to fund current operations through into the first quarter of 2025 4

Denifanstat Pipeline of Multiple Indications and Clinical Milestones 5 Therapeutic Area Indication Stage of Development Expected Milestone / Status Preclinical Phase 1 Phase 2 Phase 3 Metabolic disease NASH - F2/F3 • Phase 2b biopsy results 1Q 2024 NASH - cirrhosis • Phase 1 hepatic impairment results 1Q 2024 Dermatology Acne* • Phase 2 topline results released 2Q 2023 Oncology Solid tumors • Patient selection and trial design in FASN - dependent tumor types Recurrent GBM* • Phase 3 enrollment of 120 patients achieved in 3Q 2023; interim analysis planned * Trials conducted in China by Ascletis , who has licensed development and commercialization rights to all indications in Greater China

NASH: A Burgeoning Epidemic • No approved drugs in U.S. or Europe • Complex disease, heterogeneous patient population • Improving regulatory clarity, but liver biopsy still required • Many molecules moved forward on weak mechanism and data • Inappropriate biomarkers for mechanism that did not translate to clinical benefit • Safety: triglyceride elevations, LDL elevations, liver injury Disease challenges Drug development challenges x Designed for once - daily, oral dosing x Rigorous and de - risked development strategy x Direct DNL inhibition demonstrated in Phase 1b x Improvements observed across biomarkers in Phase 2a x Phase 2b fully - enrolled with biopsy results expected 1Q24 x Precision medicine approach to improve patient outcomes Denifanstat Patients in 2016 1 United States 85.3 million NAFL non - alcoholic fatty liver Hepatocellular carcinoma NASH non - alcoholic steatohepatitis NASH mod - adv fibrosis F2 - F3 Cirrhosis F4 11 thousand annual cases among NAFLD population 1.4 million compensated and decompensated 17.3 million 5.7 million 1 Estes, et al. 2018; http://dx.doi.org/10.1016/j.jhep.2018.05.036 DNL = de novo lipogenesis 6

Denifanstat in NASH 7

Denifanstat: Differentiated Mechanism Believed to Target Key Drivers of NASH Adapted from Wegermann et al, Clinical Liver Disease, Vol 11, No 4, April 2018, DOI: 10.1002/cld.709 Denifanstat has independent mechanisms designed to: ඹ Block steatosis via inhibiting de novo lipogenesis in hepatocytes ය Reduce inflammation via preventing immune cell activation ර Blunt fibrosis via inhibiting stellate cell activation 8

Denifanstat Showed Dose - Dependent Reduction of Liver Fat in FASCINATE - 1 Improved Key Drivers of NASH and Metabolic Health FASCINATE - 1 Liver Fat Change - 50 - 40 - 30 - 20 10 0 - 10 20 + 4.5% - 9.6% - 28.1%** Placebo n=27 25 mg n=30 50 mg n=28 Mean relative MRI - PDFF at wk12 vs baseline 1 Loomba et al, 2021 Gastroenterology. doi: 10.1053/j.gastro.2021.07.025 **p<0.005, Mean ц SEM. LSM difference versus placebo for liver fat. • Dose - finding, global, multicenter, Phase 2 trial • Oral, once - daily, 12 - week dosing FASCINATE - 1 Phase 2 study 1 • >8% liver fat and presumed fibrosis • U.S. and China 9

Denifanstat: Well Tolerated at 25/50mg Doses in FASCINATE - 1 • No dose - related significant adverse events relative to placebo • No serious AEs • Majority of AEs were Grade 1; no Grade ≥3 drug - related AEs 10

Phase 2b Biopsy Trial: Measuring Histological Improvement FASCINATE - 2 Phase 2b trial design • Biopsy confirmed F2 - F3 NASH patients • 52 weeks, 2:1 50mg or placebo, double - blind • Fully enrolled: 168 patients in U.S., Canada, and Europe • Prespecified interim analysis of the first 52 patients with MRI - PDFF >8% Primary endpoints (biopsy) Secondary endpoints 11 • NAS ≥2 points improvement w/o worsening of fibrosis OR resolution of NASH w/o worsening of fibrosis • Lead reader of liver biopsies: pathologist Pierre Bedossa MD. PhD. • Safety • Improvement in liver fibrosis ≥1 stage without worsening of NASH (Bx) • Digital AI pathology • Interim MRI - PDFF: absolute decrease, % change from baseline, % pts ≥30% (responders) x x

FASCINATE - 2 Phase 2b Interim Analysis Demographics Mean (SD) Placebo (22) Denifanstat (30) Combined Age (years) 56.8 ( 9.4) 56.1 (12.4) 56.4 (11.1) Female/Male (%) 14 (63.6%) / 8 (36.4%) 17 (56.7%) / 13 (43.3%) 31 (59.6%) / 21 (40.4%) Not Hispanic or Latino 16 (72.7%) 24 (80.0%) 40 (76.9%) Weight (kg) 97.8 (21.9) 100.9 (21.2) 99.6 (21.4) Diabetes (% T2DM) 13 (59.1%) 21 (70.0%) 34 (65.4%) F2/F3 (%) 12 (54.5%) / 10 (45.5%) 12 (40.0%) / 18 (60.0%) 24 (46.2%) / 28 (53.8%) MRI - PDFF (%) 21.78 (5.46) 17.46 (6.36) 19.29 (6.32) Fibroscan (kPa) 10.67 ( 4.07) 12.29 ( 7.33) 11.56 ( 6.04) ALT (U/L) 69.77 (42.50) 57.14 (27.55) 62.70 (35.11) AST (U/L) 51.00 (29.87) 44.43 (22.65) 47.32 (26.00) LDL (mg/dL) 111.37 (40.6) 96.29 (50.27) 102.86 (46.4) ELF 9.70 ( 0.76) 9.73 ( 0.76) 9.72 (0.75) PRO - C3 cobas® (ng/mL) 35.72 (15.71) 32.54 (11.19) 33.91 (13.28) Interim Analysis Cohort Represents Target Patient Population • Typical F2/F3 NASH population • Middle - aged • High % of diabetes • High liver fat by MRI - PDFF • Elevated liver enzymes: inflammation • Non - invasive markers of fibrosis consistent with F2/F3 12

FASCINATE - 2 Interim Results Consistent with Comprehensive Positive Readouts from FASCINATE - 1 • FASCINATE - 2 interim analysis showed consistent improvements in key drivers of NASH as observed in FASCINATE - 1 • Improvements observed in multiple biomarkers of metabolic health • LDL - cholesterol • FGF - 21 Mechanism Biomarker ඹ Steatosis Liver fat (MRI - PDFF) ය Inflammation/lipotoxicity ALT, CK - 18, ceramides ර Fibrosis PRO - C3, ELF Biomarkers replicated in FASCINATE - 2 13

Denifanstat Decreased Liver Fat Responders Correlate with Liver Biopsy Improvement ** p < 0.01, *** p < 0.001. • Denifanstat induced statistically significant reduction of liver fat • 67% (p<0.001) MRI - PDFF response rate • About half of responders decreased liver fat by ≥50% • A relative reduction of liver fat ≥ 30 % by MRI - PDFF has been shown to correlate with liver biopsy response Findings to Date ඹ Steatosis biomarker – liver fat 14

Denifastat Decreased PRO - C3 and ELF – Suggests Fibrosis Reduction • ALT decrease suggested a decrease in inflammation with denifanstat • PRO - C 3 decrease suggested a decrease of liver fibrosis with denifanstat • ELF score decrease suggested decrease of liver fibrosis with denifanstat. ELF score has prognostic value Findings to Date ය ර Inflammation and fibrosis biomarkers ALT PRO - C3 * p < 0.05 Other liver biomarkers consistent ELF score 15

Denifanstat Improved Markers of Cardiometabolic Health • LDL - cholesterol decrease: denifanstat may have cardiovascular benefit • FGF 21 increase : denifanstat may induce improvement in insulin sensitivity • Tripalmitin decrease: reflects denifanstat inhibited FASN and reduced palmitate synthesis Findings to Date Metabolic health / lipid biomarkers * p<0.05, ** p<0.01, ***p<0.001 Tripalmitin FGF21 LDL - C 16

Denifanstat Passed Planned IDMC Safety Review in FASCINATE - 2 Treatment Emergent Adverse Event (TEAE) Classification N=168 Number of Patients with Event at Stated Grade Any TEAE Gr 1: 115 (68.5%) Gr 2: 69 (41.1%) Gr 3: 10 (6.0%) Gr 4: 1 (0.6%) TEAE leading to drug/placebo discontinuation 21 Treatment Emergent Serious Adverse Event (SAE) 11 (all unrelated to study treatment) Drug/placebo - related TEAE Gr 1: 52 (30.1%) Gr 2: 25 (14.9%) AE data as of 3 April 2023 Sagimet is blinded to data • All randomized subjects: blinded data set including active and placebo • Majority of AEs to date were Grade 1 or 2; no Grade ≥3 drug - related AEs • A planned safety review of unblinded data from all 168 patients conducted by Independent Data Monitoring Committee – no concerns FASCINATE - 2 Phase 2b - Blinded data set 17

NASH Development Program 18

Progression from Phase 2b to Phase 3 Phase 2b – baseline Fibrosis stage Phase 2b – 26 weeks Non - invasive interim Phase 2b – 52 weeks Histology Phase 3 Fibrosis endpoint - human Expect to use Phase 2b results including AI pathology scores to design and power Phase 3 Primary endpoints • NAS ≥2 improvement w/o worsening of fibrosis; or NASH resolution w/o worsening of fibrosis • Safety Secondary endpoints • Fibrosis ≥1 stage improvement w/o worsening of NASH • Digital AI pathology Interim cohort F2 – 46.2% F3 – 53.8% 19 Interim results released Nov 2022 Biopsy results expected 1Q 2024 Startup activities expected 2024 Enrollment completed Sep 2022

We Believe Denifanstat is Differentiated in the Evolving NASH Landscape Mechanism FASN inhibitors THRß agonists FGF - 21 GLP - 1 agonists PPAR agonists ACC inhibitors FXR agonists Category DNL pathway Nuclear receptor Growth factor GLP - 1 Nuclear receptor DNL pathway Nuclear receptor Route Oral Oral Injectable Injectable Oral Oral Oral Status Phase 2 ongoing Phase 3 complete Phase 2 complete Phase 2 complete Phase 3 ongoing Phase 2 complete Phase 3 complete Challenges • Pending biopsy results • Selectivity for beta isoform critical to avoid potential heart and bone safety issues • Injectable • Nausea and diarrhea • Potential neutralizing antibodies • COGS • GI side effects including nausea • Lack of fibrosis improvement to date • Weight gain, edema, GI side effects, anemia • Combinations only • MOA causes triglyceride increases • Lack of fibrosis improvement as monotherapy • Mixed results from several programs • MOA causes pruritus and LDL - cholesterol increases 20

Precision Medicine: Blood Tests May Lead to Improved Patient Outcomes 1 Signature has 6 metabolites: ursodeoxycholic acid, DL - 2 - aminocaprylic acid, sarcosine, glycoursodeoxycholic acid, D( - ) - 2 - aminobutyric acid, PC(0 - 18:0/22:4). Accuracy 79%, PPV 88%, NPV 63%. Blood test for predictive marker denifanstat denifanstat • NASH is a multi - faceted disease and patients may benefit from being matched with optimal treatments • Two approaches using blood tests are undergoing further evaluation • Drug response: 1 - 2 months after taking drug, tripalmitin identifies patients responding to drug treatment • Predictive marker: before taking drug, signature of 6 blood metabolites enriches for responders 1 Blood test for drug response (e.g. tripalmitin) Clinical response rate denifanstat Clinical response rate On - treatment 1 - 2 months Pre - treatment 21

Strong Monotherapy Opportunity for Denifanstat in NASH Expansion as backbone of combinations Illustrative potential combo mechanisms FXR GLP - 1, PPAR, FGF21 DGAT2 →→ThR HSP47, integrins NLRP3 Chemokines, ARO - HSD x Oral, once - daily tablet ideal for chronic administration • Tablets generally more affordable than complex biologics x Potential to treat broad patient population • Including those with thyroid challenges x Novel mechanism that acts directly upon liver x Encouraging safety profile to date Denifanstat data support success as first line monotherapy • Denifanstat’s potential x Complementary to other mechanisms x Potential for fixed dose combinations with other oral medications x Preclinical combination studies ongoing • NASH agents: anti - fibrotic, other metabolic agents • Co - morbidities: diabetes and other cardiovascular agents Broaden market opportunity through combinations with denifanstat as backbone 22

Additional Expansion Opportunities in NASH • Compensated cirrhotic patients (NASH F4) • Denifanstat directly targets stellate cells • Hepatocytes continue to be functional, and patients frequently have increased liver fat • Next steps • Characterize PK profile in patients with impaired hepatic function – Phase 1 results in 1Q 24 • Positive impact on fibrosis in FASCINATE - 2 • Phase 2b/3 trial in NASH - F4 • Pediatric NASH • 23% of children with NAFLD have NASH at the time of diagnosis • Next steps • Compile safety data across all denifanstat studies in young adults including FASCINATE - 2 • Nonclinical toxicology study in juvenile animals – plan to initiate in 2024 • Phase 2 trial in pediatric NASH 1 Estes, et al. 2018; http://dx.doi.org/10.1016/j.jhep.2018.05.036 F compensated and decompensated 23

Other Indications 24

FASN Hyper - Activity Plays a Key Role in Multiple Diseases Beyond NASH Cancer cell Membrane synthesis, intracellular signaling, protein modification FASN in cancer 1. Supports tumor survival 2. Enables tumor proliferation 3. Establishes resistance to drugs FASN in NASH 1. Drives steatosis 2. Activates pro - inflammatory cells 3. Activates stellate cells leading to fibrosis Sebocyte Sebum production FASN in acne 1. Sebum production 2. Sebum composition 25
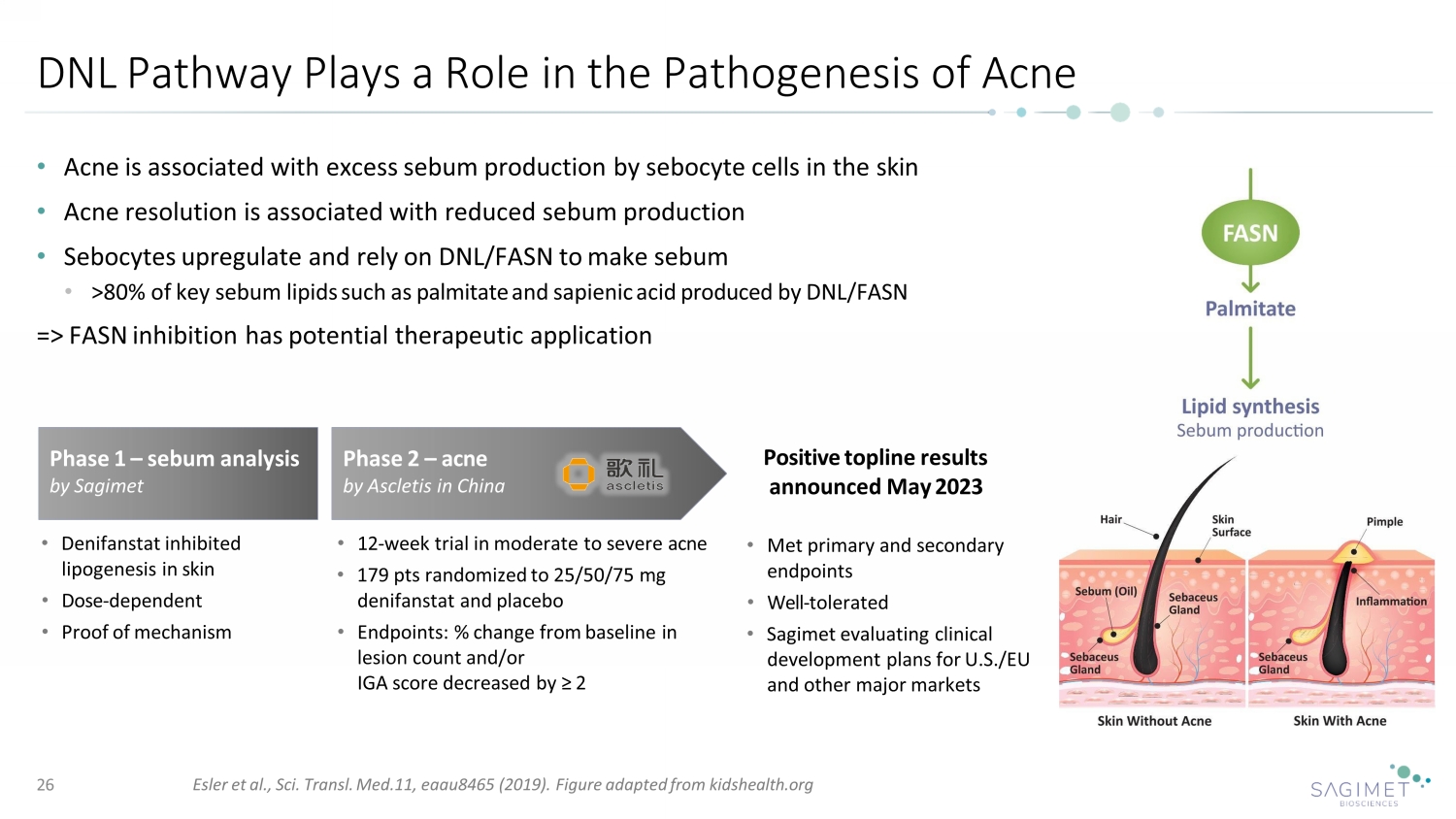
DNL Pathway Plays a Role in the Pathogenesis of Acne • Acne is associated with excess sebum production by sebocyte cells in the skin • Acne resolution is associated with reduced sebum production • Sebocytes upregulate and rely on DNL/FASN to make sebum • >80% of key sebum lipids such as palmitate and sapienic acid produced by DNL/FASN => FASN inhibition has potential therapeutic application Esler et al., Sci. Transl. Med.11, eaau8465 (2019). Figure adapted from kidshealth.org Phase 2 – acne by Ascletis in China Phase 1 – sebum analysis by Sagimet Positive topline results announced May 2023 • Denifanstat inhibited lipogenesis in skin • Dose - dependent • Proof of mechanism • 12 - week trial in moderate to severe acne • 179 pts randomized to 25/50/75 mg denifanstat and placebo • Endpoints: % change from baseline in lesion count and/or IGA score decreased by ≥ 2 • Met primary and secondary endpoints • Well - tolerated • Sagimet evaluating clinical development plans for U.S./EU and other major markets 26

FASN is Integral to Tumor Cell Proliferation and Survival Reprogramed metabolism is one of the hallmarks of cancer • Certain cancers are dependent on DNL/FASN for proliferation especially downstream of driver oncogenes • eg. KRASM in non - small cell lung cancer (NSCLC) • Strategy – > exploit this vulnerability using FASN inhibition in the combination setting to cause death Dietary fatty acids cannot compensate for de novo synthesized palmitate Specific oncogenic drivers are FASN - dependent Prevents lipid peroxidation and stress induced death Palmitate RTK e.g. MET, VEGFR Saturated fatty acids for lipid rafts and membranes Protein modification and localization/ function Receptor localization and signaling Acetyl - CoA Malonyl - CoA pS6 mTOR AKT PI3K KRAS - 4A B - tubulin WNT Lipid rafts FASN Completed Phase 1 provides foundation • 136 patients received denifanstat • Heavily pretreated Phase 1 population • Recommended Phase 2 dose defined • Promising clinical activity consistent with proposed mechanism • KRASM NSCLC patients had significantly longer duration on study with denifanstat than KRASWT (p<0.02), and 91% KRASM had stable disease FASN - dependence 27

FASN - Dependent Tumor Types Identified that Meet Core Criteria Program focused on 4 selected tumor types Preclinical ongoing • Combination with KRAS inhibitor in mouse models x Encouraging Phase 1 results with denifanstat Translational ongoing • Patient selection bioinformatics x Positive preclinical results Phase 1 pending start • Investigator Sponsored Trial at Weill Cornell, in combination with enzalutamide x Positive preclinical results Phase 3 ongoing • By Ascletis in China, in combination with bevacizumab x Positive Phase 2 investigator sponsored trial results NSCLC KRASM HCC FASN - dependent Prostate FASN - dependent GBM If positive, favor clinical collaboration with a KRASM industry partner If patient selection is tractable, Sagimet would sponsor a clinical study Phase 1 results will inform clinical decision by Sagimet Phase 3 results will inform clinical decision by Sagimet x FASN - dependent mechanism x Preclinical or clinical POC shown x Unmet clinical need x Tractable clinical path including patient selection Core criteria Tumor type Status Next milestone GBM (glioblastoma), HCC (hepatocellular carcinoma), KRASM (mutant KRAS), NSCLC (non small cell lung cancer) 28

Strong Financial Position and Intellectual Property Portfolio Financial highlights Nasdaq: SGMT x Upsized IPO completed in July 2023 raised $96.4 million of gross proceeds x Cash and equivalents expected to fund current operations into the first quarter of 2025 Strong patent estate x Composition of matter for denifanstat: 2032 x Issued in all key commercial territories x Opportunities to lengthen exclusivity via Hatch - Waxman and synthesis/formulation applications 29

Denifanstat Pipeline of Multiple Indications and Clinical Milestones 30 Therapeutic Area Indication Stage of Development Expected Milestone / Status Preclinical Phase 1 Phase 2 Phase 3 Metabolic disease NASH - F2/F3 • Phase 2b biopsy results 1Q 2024 NASH - cirrhosis • Phase 1 hepatic impairment results 1Q 2024 Dermatology Acne* • Phase 2 topline results released 2Q 2023 Oncology Solid tumors • Patient selection and trial design in FASN - dependent tumor types Recurrent GBM* • Phase 3 enrollment of 120 patients achieved in 3Q 2023; interim analysis planned * Trials conducted in China by Ascletis , who has licensed development and commercialization rights to all indications in Greater China
Exhibit 99.2

Sagimet Biosciences Announces Completion of Enrollment of 120 Patients for Phase 3 Clinical Trial by Its Partner Ascletis of Denifanstat Combined with Bevacizumab for Treatment of Recurrent Glioblastoma
San Mateo, Calif., September 26, 2023 – Sagimet Biosciences Inc. (Nasdaq: SGMT), a clinical-stage biopharmaceutical company developing novel therapeutics targeting dysfunctional metabolic pathways, today announced that its license partner, Ascletis Bioscience Co. Ltd. (Ascletis), has enrolled 120 patients in its Phase 3 registration clinical trial of denifanstat combined with bevacizumab for treatment of recurrent glioblastoma (rGBM). Ascletis anticipates that this number of study subjects will provide sufficient events for its planned interim analysis of progression-free survival (PFS). Denifanstat is an oral, selective small molecule inhibitor of fatty acid synthase (FASN), a key enzyme which regulates de novo lipogenesis (DNL). Sagimet licensed the rights to develop and commercialize denifanstat in the People’s Republic of China, Hong Kong, Macau and Taiwan to Ascletis in January 2019.
Sagimet’s FASCINATE-2 Phase 2b clinical trial for denifanstat in liver biopsy-confirmed F2-F3 nonalcoholic steatohepatitis (NASH) patients is fully enrolled and biopsy results are expected in the first quarter of 2024. Sagimet also expects to report Phase 1 clinical trial results characterizing the pharmacokinetic profile of denifanstat in patients with impaired hepatic function in the first quarter of 2024.
“We congratulate Ascletis on achieving this important patient enrollment milestone in its Phase 3 clinical trial of denifanstat being conducted in China in patients with recurrent glioblastoma,” stated David Happel, Chief Executive Officer of Sagimet. “Sagimet looks forward to reporting biopsy results and other key endpoints from our Phase 2 FASCINATE-2 trial for denifanstat in patients with NASH in the first quarter of 2024, and, if the data is positive, potentially advancing the program into a registrational Phase 3 trial.”
About Sagimet Biosciences
Sagimet is a clinical-stage biopharmaceutical company developing novel therapeutics called fatty acid synthase (FASN) inhibitors that target dysfunctional metabolic pathways in diseases resulting from the overproduction of the fatty acid, palmitate. Sagimet’s lead drug candidate, denifanstat, is an oral, once-daily pill and selective FASN inhibitor in development for the treatment of nonalcoholic steatohepatitis (NASH), for which there are no treatments currently approved in the United States or Europe. Denifanstat is currently being tested in FASCINATE-2, a Phase 2b clinical trial in NASH with liver biopsy as the primary endpoint. For additional information about Sagimet, please visit www.sagimet.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this press release, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding: the expected timing of the presentation of data from ongoing clinical trials, Sagimet’s clinical development plans and related anticipated development milestones, Sagimet’s cash and financial resources and expected cash runway. These statements involve known and unknown risks, uncertainties and other important factors that may cause Sagimet’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, these statements can be identified by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions.
The forward-looking statements in this press release are only predictions. Sagimet has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that Sagimet believes may affect its business, financial condition and results of operations. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond Sagimet’s control, including, among others: the clinical development and therapeutic potential of denifanstat or any other drug candidates Sagimet may develop; Sagimet’s ability to advance drug candidates into and successfully complete clinical trials, including its FASCINATE-2 Phase 2b clinical trial; Sagimet’s relationship with Ascletis, and the success of its development efforts for denifanstat; the accuracy of Sagimet’s estimates regarding its capital requirements; and Sagimet’s ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of Sagimet’s most recent filings with the Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in these forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, Sagimet operates in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that Sagimet may face. Except as required by applicable law, Sagimet does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Contact:
Robert Uhl
Managing Director, ICR Westwicke
858.356.5932
robert.uhl@westwicke.com
